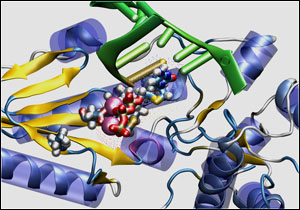
Lamivudine complexes with nucleic acids (green) in HBV
polymerase, highlighting residues related to drug resistance
Clinical Trials
SeqHepB database, underpinned by an extensive patent portfolio of HBV mutations, is the first comprehensive database that includes genetic, clinical and phenotypic details for chronic HBV. It allows rapid genomic analysis and cross-link genetic data with clinical information on an individual as well as cohort basis.
The SeqHepB database can be used by pharmaceutical companies in selecting patients for clinical trials. It can potentially assist in screening patients for eligibility for clinical trials and expanded treatment access programs.
Data in the SeqHepB database can be used for the following purposes:
- Epidemiological mapping for clinical trials based on regional, provincial or national level
- Surveillance of treatment efficacies or emergence of novel mutations associated with drug resistance
- Correlation of clinical data, HBV-related pathology test results, viral sequence details, and drug sensitivity values (in vitro phenotype data)
- Selection of patient cohorts with a specific set of criteria at the regional, provincial and national level
All material copyright Evivar Medical Pty. Ltd. All rights reserved